Boosters
The CDC now recommends that people whose immune systems are compromised moderately to severely should receive an additional dose of mRNA COVID-19 vaccine after the initial 2 doses.
Frequently Asked Questions
• Been receiving active cancer treatment for tumors or cancers of the blood
• Received an organ transplant and are taking medicine to suppress the immune system
• Received a stem cell transplant within the last 2 years or are taking medicine to suppress the immune system
• Moderate or severe primary immunodeficiency (such as DiGeorge syndrome, Wiskott-Aldrich syndrome)
• Advanced or untreated HIV infection
• Active treatment with high-dose corticosteroids or other drugs that may suppress your immune response
People should talk to their healthcare provider about their medical condition, and whether getting an additional dose is appropriate for them.
**NOTE: The CDC does not recommend additional doses or booster shots for any other population at this time.**
There is no required proof outside of self-attestation. Self-attestation is a statement or written document to self-proclaim that you do meet the qualifications to receive a booster.
Self-Attestation for Moderately to Severely Immunocompromised People
Autoatestación para personas con sistema inmunitario que se encuentra comprometido de estado moderado a grave
If it has been at least 28 days (4 weeks) or more since your 2nd dose then you can receive a booster shot of either Pfizer or Moderna.
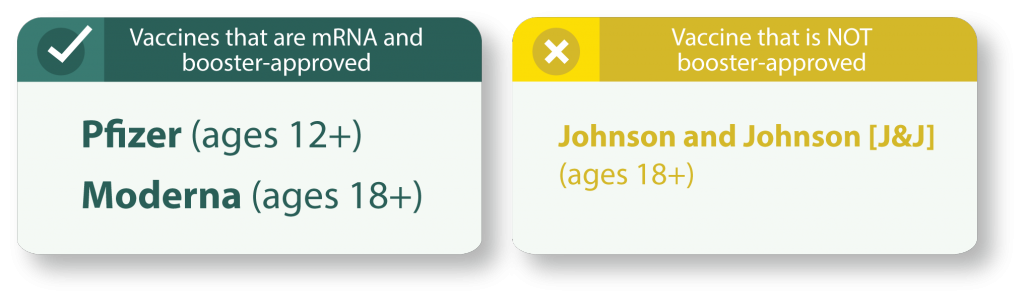
For people who received either Pfizer-BioNTech or Moderna’s COVID-19 vaccine series, a third dose of the same mRNA vaccine should be used. A person should not receive more than three mRNA vaccine doses. If the mRNA vaccine product given for the first two doses is not available or is unknown, either mRNA COVID-19 vaccine product may be administered.
The FDA’s recent EUA amendment only applies to mRNA COVID-19 vaccines, as does CDC’s recommendation.
Emerging data have demonstrated that immunocompromised people who have low or no protection following two doses of mRNA COVID-19 vaccines may have an improved response after an additional dose of the same vaccine. There is not enough data at this time to determine whether immunocompromised people who received the Johnson & Johnson’s Janssen COVID-19 vaccine also have an improved antibody response following an additional dose of the same vaccine.
There is limited information about the risks of receiving an additional dose of vaccine, and the safety, efficacy, and benefit of additional doses of COVID-19 vaccine in immunocompromised people continues to be evaluated. So far, reactions reported after the third mRNA dose were similar to that of the two-dose series: fatigue and pain at injection site were the most commonly reported side effects, and overall, most symptoms were mild to moderate.
NOTE: The FAQ above can be found on the CDC website at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
Facts Sheets for Patients
Pfizer Patient Fact Sheet: https://www.fda.gov/media/144414/download
Moderna Patient Fact Sheet: https://www.fda.gov/media/144638/download